A drug used to treat a type of pneumonia has been recalled after tests showed it could be as deadly as the infection it was treating.
AvKARE has recalled one lot of Atovaquone Oral Suspension after third-party testing revealed that the drug may be contaminated with Bacillus cereus.
As for what this means, the risk description in the AvKARE recall notice reads: “In immunocompromised populations, which are most at risk, there is a reasonable probability that microbial contamination of atovaquone oral suspension may cause disseminated life-threatening infections such as endocarditis.” and a necrotizing soft tissue infection. ”
The Mayo Clinic describes endocarditis as “inflammation of the heart.” inner wall of heart chamber And the valve. The Johns Hopkins School of Medicine says that “necrotizing soft tissue infection” is a “serious and life-threatening condition that requires immediate treatment to prevent infection.” destroys skin, muscle, and other soft tissue”
AvKARE has extracted only Atovaquone Oral Suspension, USP 750mg/5mL with lot number AW0221A and expiration date August 2025. The NDC and UPC number is 5026808612. However, the NDC number is divided into his 3 parts after his 5th number and 8th number. The drug was shipped to wholesalers nationwide from March 18th to March 21st.
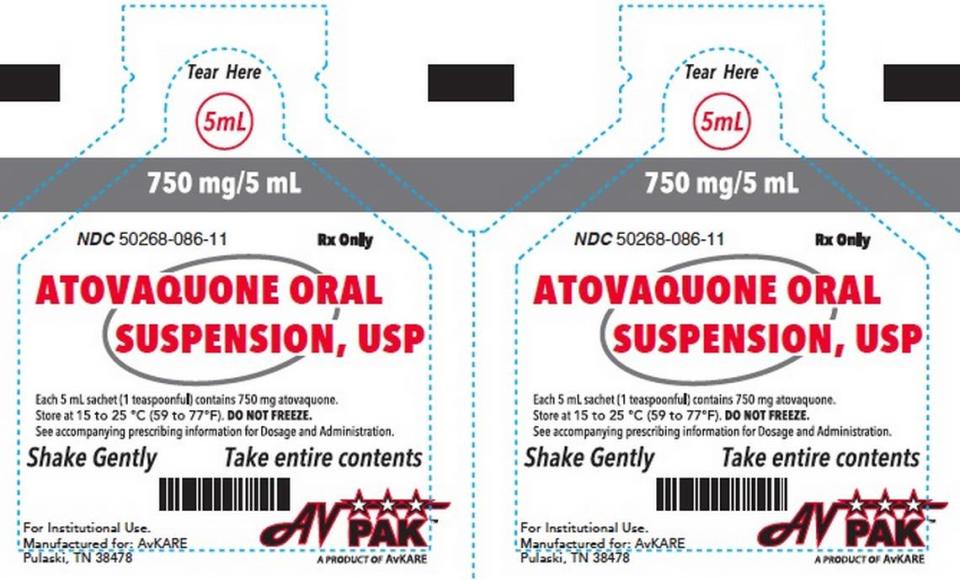
The medicine arrived in a carton. If you have this medication, stop using it immediately. Then throw it away or return it to the store for a refund.
Contact your health care professional if you have a medical reaction to your medicine.after that Contact FDA’s MedWatch Program Online or at 800-332-1088.
For questions regarding the recall, please contact AvKARE at 855-361-3993 or Drugsafety@avkare.com, Monday through Friday, 9 a.m. to 5 p.m. Eastern Time.