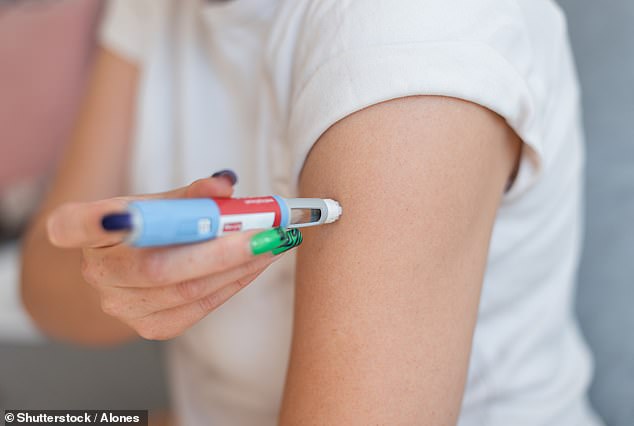
Ten people have died and 100 have been hospitalized after taking a counterfeit version of Ozempic made in a pharmacy, its manufacturer has revealed.
These compound weight loss drugs are often inexpensive and have been available in “medispas” and pharmacies for the past two years during a national shortage of branded drugs.
Ozempic’s maker, Novo Nordisk, claims these off-brand versions of its drug are manufactured with little regulation or oversight, increasing the risk of people getting sick after using them.
Few details about the patients were disclosed, including their location, age, and side effects they suffered.
However, the FDA notes that reactions to the combined version are often associated with overdose or accidentally administering the wrong dose, which can result in hospitalization for complications such as nausea, vomiting, diarrhea, abdominal pain, and constipation. said.
Experts have previously warned that the drug can cause severe hypoglycemia, putting people at risk of seizures and coma if taken in excess.
Novo disclosed the incident last month after asking the FDA to ban a combination version of its drugs Ozempic and Wigovy. It is said to be too complicated to make at a pharmacy.
The weight-loss drug, which uses the drug Semaglut, has been in short supply in the United States for more than two years after its use skyrocketed after Hollywood promoted it with claims that weekly injections were all it took to lose weight.

Combined versions of Ozempic made in pharmacies are associated with deaths
The FDA still needs to make a final decision on whether to ban the combined form of Ozempic and semaglutide, the active ingredient in Wegovy.
U.S. regulations allow pharmacies to manufacture proprietary or compounded versions of branded drugs when they are out of stock to fill prescriptions.
Supporters say this is necessary to ensure people continue to receive potentially lifesaving drugs and adhere to their doses.
However, against the practice of Ozempic, Novo Nordisk CEO Lars Fluergaard Jorgensen said it was “incomprehensible” that people in the United States were being allowed to self-inject a product that was not regulated, approved or tested.
“I’m very devastated,” he said in an interview with Reuters, before adding: “I think this is something that will change over time.”
Novo Nordisk said the counterfeit versions were often sold online and through so-called “health spas” rather than through the official supply chain.
The company is also investigating a number of compounded products that have been identified as having multiple safety concerns, although details have not been disclosed.
Novo Nordisk’s tally is lower than that of the FDA, which said it had received 346 reports of adverse events related to combination semaglutide by the end of August this year. The number of deaths was not disclosed.
This is lower than the number of deaths reported in the FDA’s Adverse Event Reporting System (FAERS) related to semaglutide, the drug used in Wigovy and Ozempic (94 as of early September of this year). .
This system only suggests a link, but does not conclusively prove that the death was caused by the drug.






Trish Webster, 56, from Australia, died after injecting herself with Ozempic to lose weight in preparation for her daughter’s wedding. She is pictured above with her husband Roy
There were also 68 deaths linked to tirzepatide, the drug used in the Mounjaro and Zepbound weight loss injections.
Novo Nordisk is currently ramping up production to meet demand in the US, with Wegoby and Ozempic all back in stock across the country.
However, these drugs are still on the FDA’s shortage list, allowing pharmacies to continue manufacturing combination drugs.
“This is an ongoing dialogue with the FDA,” said Novo Nordisk Chief Financial Officer Carsten Munk Knudsen.
“I don’t want to speculate on whether we’re completely off the missing list, but this is a first step and we’re hopeful that we’ll be off the list in the future.”
According to Penn Medicine, nearly 5 million Americans were prescribed semaglutide in 2023, and 4 in 10 were using semaglutide for weight loss.
One month of Ozempic costs $935.77 per month out-of-pocket, while one month of Wegovy can cost up to $2,000.