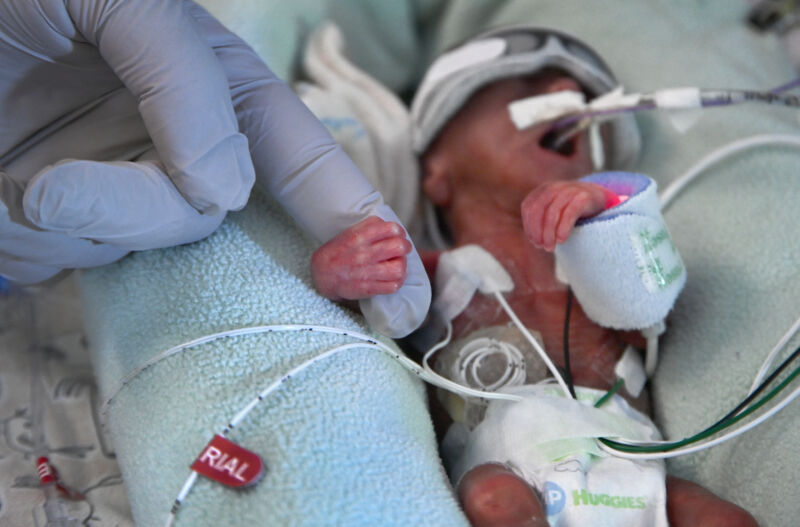
The Food and Drug Administration Alert healthcare provider To discourage the use of probiotics containing live bacteria or yeast in premature babies, authorities have launched an investigation into the death of a premature, low birth weight baby born at an unnamed hospital in July who was given such products. Receive it.
The infant developed sepsis due to bacteria in a probiotic product (Evivo with MCT Oil by Infinant Health) and subsequently died.
In a statement to Als, the FDA said it quickly investigated the death after receiving the initial report on July 31. “Infant deaths are particularly tragic, and determining causation in premature infant deaths can be particularly complex,” an FDA spokesperson said. The agency reviewed the case’s medical records and laboratory tests and collected clinical and product samples for analysis.
“Genome sequencing data analysis conducted by FDA in September 2023 found that the probiotic bacteria present in Evivo, which contains MCT oil, is genetically consistent with bacteria isolated from infant blood.” said.
Bacteria contained in products and detected in infants are Bifidobacterium longum Subspecies infant.
The FDA did not respond to Ars’ questions about the state or hospital where the death occurred.
On Friday, the FDA issued a warning to health care professionals about the dangers of probiotics in infants, especially extremely vulnerable preterm infants. The agency noted that this is not the first time this type of incident has occurred. There have been reports of infections and sepsis in infants due to the use of probiotics, including: bacteria and yeast.
“FDA also reminds health care professionals that FDA has not approved any probiotic product for use as a drug or biological product in infants,” the agency wrote in the warning. .
Unproven and dangerous treatments
Probiotics are usually not recommended for infants, especially vulnerable infants. The American Academy of Pediatrics does not recommend them. “Given that there are no FDA-regulated pharmaceutical-grade products in the United States, conflicting safety and efficacy data, and the potential for harm to highly vulnerable populations, current evidence suggests that does not support the routine and universal administration of probiotics to preterm infants, especially when birth weight is less than 1000 g.” Yet, 2021 ReportIt turns out that about 10 percent of extremely premature babies in the United States receive some kind of probiotic preparation while receiving specialized care in the hospital.
Although the FDA has attempted to inform health care professionals about the dangers of probiotics, the FDA also Letter of Reprimand to Manufacturer of Evivo with MCT Oil: Infinant Health, IncThe agency said the California-based company was clearly marketing unapproved products sold as dietary supplements for use in preventing serious illness in highly vulnerable preterm infants. criticized. Additionally, the agency took aim at the company, which advertised the supplement as being “designed specifically for use in medical settings.”[d]Specially designed for infants in the NICU[.]“The NICU is a neonatal intensive care unit, where the most vulnerable newborns, many born prematurely, receive specialized intensive care.
Preterm infants are infants born before 37 weeks of gestation and are at increased risk for morbidity and mortality, the FDA notes. “With their digestive systems not fully mature, preterm infants have a more permeable intestinal lining, often referred to as a ‘leaky gut,’ they have problems with motility, and when they ingest live microorganisms, they develop opportunistic infections.” and can cause sepsis.”
Infinant Health claims its product can prevent necrotizing enterocolitis, a life-threatening inflammation of the colon that affects newborns, especially premature and low birth weight infants. However, published data does not support that claim, and Infinant Health’s products have not undergone premarket review by the FDA for safety and efficacy. The FDA has also not approved it for use in clinical trials.
The FDA’s warning letter to Infinant Health states that under federal regulations, Evivo with MCT oil is both an unapproved new drug and an adulterated food. However, it is unclear whether Infinant was aware of this beforehand.
multiple meetings
The FDA’s warning letter to Infinant Health says the company met with the agency in early July, before the infant’s death. During the meeting, the FDA expressed concerns about the company’s products.
“FDA, at a meeting between FDA’s Center for Food Safety and Applied Nutrition (CFSAN) and the Food Additive Safety Administration’s Division of Food Ingredients and Infant Health on July 7, 2023, “We have communicated safety concerns related to the use of the drug,” the FDA wrote. “Specifically, the FDA believes that the clinical studies Infinant cited in its presentation to the FDA were not robust enough to support safety because they were short-term, poorly designed, and difficult to interpret without proper controls.” I questioned whether or not it was sexual.”
FDA repeatedly refused to answer Ars’ questions about this meeting, including why it happened and whether it led to FDA action.
The FDA’s letter to Infinant says the agency and the company met again in September. FDA suggests that rather than listening to regulators’ concerns about its products, Infinant may be following suit and making further unsubstantiated claims that FDA may not be aware of. It seemed like he was doing it.
“During your meeting with FDA on September 21, 2023, your company highlighted the following benefits to FDA representatives: B. Longham’s subspecies iNfantis in Evivo uses MCT oil in preterm infants in neonatal intensive care units to reduce the incidence of necrotizing enterocolitis. These statements suggest that similar claims may exist on other labels and promotional materials to which FDA does not have access. ”
Infinant Health did not immediately respond to Als’ questions.
A company spokesperson responded. To CBS NewsHowever, Infinant Health plans to continue distributing Evivo powder products for consumer purchase and is “working with the FDA to approve the use of our MCT oil products in hospital settings.” He said he intended to do so.