The Government of Prince Edward Island (PEI) has announced that it has become the 11th jurisdiction in Canada to implement a biosimilar “switch” or transition policy. statement From Canadian biosimilars.
This policy requires enrollment in the PEI Pharmacare Program; Specific reference biological products To move to a biosimilar version (table). Additionally, all new patients requiring treatment with these agents will need to start receiving the biosimilar. This new policy will allow PEI to fund more new drug treatments, bring innovation to the province’s health care system and improve patient care.
If a patient is unable to make an appointment with a prescriber by the deadline, PEI’s government website has an exception form for health care providers to fill out. Once approved, the special authorization for the reference drug will be extended for one month from the patient’s next appointment. Reservations must be scheduled by September 30, 2024.
“Biosimilars Canada and its member companies are committed to supporting Health Minister Mark MacLean and the Government of Prince Edward Island in implementing biosimilar switching policies to support the long-term sustainability of the province’s public drug program and health care system. Congratulations on doing so,” said Jim Keown. , President of Canadian Biosimilars.
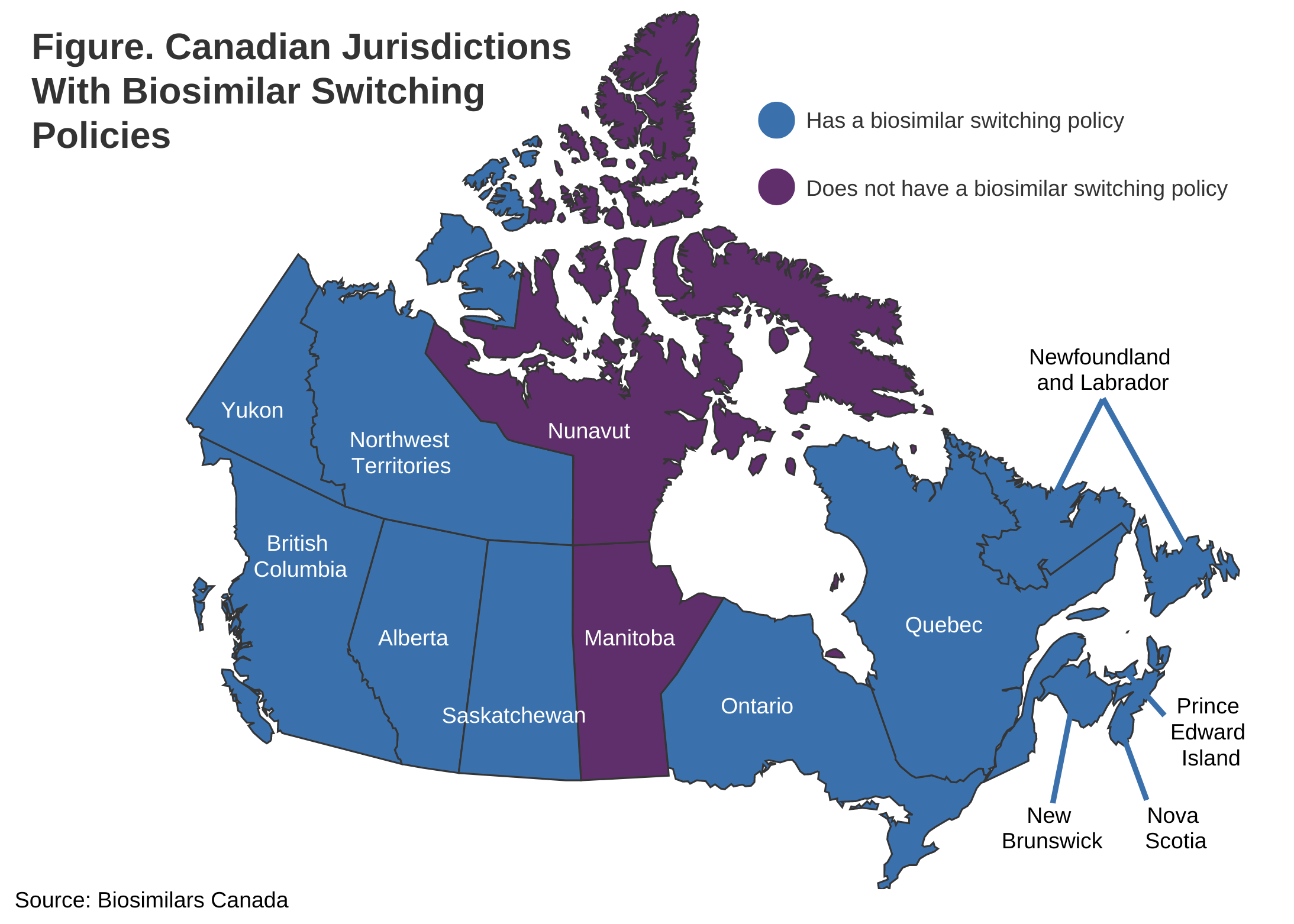
PEI policy will be published in British Columbia (May 2019), Alberta (December 2019), New Brunswick (April 2021), Quebec (July 2021), and the Northwest Territories (December 2021). ), Nova Scotia (February 2022), Saskatchewan (October 2022), Ontario (December 2022), Newfoundland and Labrador (March 2023), Yukon (March 2023) Month) (shape).
Healthcare providers have until June 30, 2024 to inform patients on the selected reference drug that their product will be changed, explain what a biosimilar is, and the safety of the transition. need to be educated. After the deadline, the reference medicine is no longer covered by the public health program. As new biosimilars become available, this initiative PEI Pharmacare Formulas.
Manitoba and Nunavut are the only provinces without biosimilar transition policies.
The topic of switching patients from reference products to biosimilars has raised concerns about whether the product transition changes safety or efficacy outcomes. However, none of the states with switching policies have had an impact on clinical outcomes, and both the European Union and the United Kingdom have declared all biosimilars interchangeable with the reference product.
In Health Canada’s Biosimilars Fact Sheet, Health Canada states that “Patients and healthcare providers should be confident that biosimilars are effective and safe for each approved indication.” “We do not anticipate differences in efficacy or safety due to changes in routine use among Health Canada.” Biosimilars and their reference biologics in approved indications. ”
Biosimilars have revolutionized the treatment of several chronic and oncological diseases, but treating a patient for one year can cost between $10,000 and $25,000, sometimes more. , which can place a significant financial burden on the healthcare system and place a strain on healthcare institutions. medicine budget.
Manitoba and Nunavut are the remaining jurisdictions without biosimilar transition policies. However, Manitoba remains the only jurisdiction with a public health plan.
Nunavut is the smallest of the territories, with a population of approximately 38,000 people, and most of its residents are covered by federal drug programs. Although Nunavut does not have the resources to maintain its own prescription system and does not provide prescription drug coverage to uninsured residents, it does provide coverage for products covered by the federal Uninsured Health Benefits Program prescription system. We offer refunds. Due to the unique circumstances of the jurisdiction, a biosimilar transition policy will never be necessary in Nunavut.
