Discover how MilliporeSigma expands its tissue diagnostic antibody portfolio to better classify gliomas and other tumors of the nervous system
All cancers are characterized by a unique set of genetic alterations, including amplifications, rearrangements, translocations, or indels (additions or deletions). These changes drive tumor development and can be used as markers to define tumor type and developmental stage, and even predict metastatic risk and potential response to therapy. These cancer biomarkers (or mutation markers) are currently the subject of intense research because they provide clinicians with valuable information to help diagnose, classify, and track tumor prognosis. Moreover, these markers can inform precise therapeutic approaches that are individualized for individual patients and tumor types.
In this article, we speak with experts at Millipore Sigma to learn that new antibodies developed using the revolutionary Cell Marque™ Tissue Diagnostics technology are being used by neuropathologists to target gliomas, gliomas, and brain glial cells (supporting cells). cells) to help classify brain tumors. central nervous system.
Our goal is to remove the guesswork and bring confidence in diagnosis.
Brent Heller
millipore sigma
Bringing confidence to tissue diagnosis
Brent Heller, Millipore Sigma
“Mutational markers are an important step towards better distinguishing between malignancies,” said Brent Heller, commercial director of histological diagnostics division, Western North America. However, it is not without its challenges. “At MilliporeSigma, we see value in these unique targets, but developing accurate assays that are easy to use in the lab remains a challenge,” explains Heller. “You need mutant tissues to develop assays, but you need assays to identify tissues. So which comes first?”
To meet this challenge, researchers must use alternative tests to confirm tissue identity. One method for her to detect mutational markers is by sequencing the DNA of tumor samples. However, this approach is expensive and requires relatively large amounts of tissue, which is not always available, especially for brain and spinal cord tumors. Additionally, this approach can also yield false-negative results when there are few tumor cells in the sample compared to the number of non-neoplastic cells. Immunohistochemistry (IHC) using antibodies targeting specific mutations is a convenient and cost-effective alternative screening method that provides genetic information related to tumor morphology.
With Cell Marque Tissue Diagnostics technology, clinicians can now deliver the experiments they need to their clients in a more cost-effective and time-effective manner. “We invest time and energy upfront to make sure these mutation assays are specific,” Heller says. Several different approaches are used in Millipore Sigma’s tissue diagnostic development process, including sequencing the tissue itself, working with partners in his lab on known mutation cases, and using known cell lines with the same mutation. .
A key part of the company’s approach is the custom design and manufacture of rabbit monoclonal antibodies using proprietary B-cell cloning technology. The traditional method of producing monoclonal antibodies is to inoculate rabbits or mice with the molecule of interest. Researchers then collect and isolate the resulting antibodies and test their ability to bind target molecules with high affinity. However, there are many variations in this process. “The animal’s immune system is trying to break down the target molecule into fragments or antigens,” explains Heller. “You can’t control it.”
In addition to being an animal-free process, B-cell cloning allows MilliporeSigma to precisely input the amino acid sequence of the mutation of interest and produce highly specific products. This is essential for accurate diagnostic testing. “Our goal is to take the guesswork out and give you confidence in your diagnosis,” Heller says. “B-cell cloning helps to produce monoclonal cell lines for stable production of antibodies, ensuring sustainable supply and performance.
In traditional classification, diagnostic practice suffers greatly from poor predictive performance and reproducibility.
Dr. Chin Su
millipore sigma
Reliable antibodies for glioma classification
Traditional classification is based on: –
system:
Me.astrocytes
ii. Oligodendroglia
iii. Oligoastrocytes
Histology:
Me. Grade II (benign)
ii. Grade III (low grade)
iii. Grade IV (high grade)
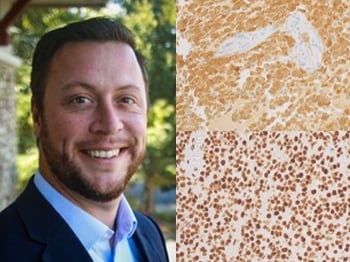
One of the team’s newest antibodies, IDH1 R132H (MRQ-67), is helping clinicians identify and classify adult-onset diffuse glioma.
“Traditionally, diffuse gliomas were classified primarily based on morphology. Microscopic observation alone divided them into three lineage types and three histologic grades,” said MilliporeSigma staff pathology. Academician Dr. Qin Su explains. “With this classification, diagnostic practice has suffered greatly from poor predictive performance and reproducibility.”
However, since 2016, the WHO classification of gliomas has integrated morphology along with the status of the following key molecular alterations common in these types of tumors: IDH1 mutations, presence of ATRX. or loss, and 1p/19q co-deletion.
As an example, IDH1 encodes an enzyme called isocitrate dehydrogenase 1 (IDH1), which is part of the citric acid cycle in all cells under normal conditions. Mutations in IDH1 are common in several malignancies, including diffuse and anaplastic gliomas and secondary glioblastomas. An arginine to histidine substitution (R132H) at codon 132 of this protein is found in more than 90% of adult-onset gliomas, but is also present in several rare types of myeloid leukemia.
MilliporeSigma quickly realized there was a gap in the market. in vitro We developed a diagnostic antibody for glioma and used our B-cell cloning technology to develop a monoclonal antibody (designated MRQ-67) that targets the R132H mutation of IDH1. This antibody has been shown to specifically recognize mutations in both adult-onset glioma and myeloid leukemia. Furthermore, MRQ-67 outperformed a widely available murine monoclonal antibody targeting the same mutation. Download Comparative Study >>
Alongside MRQ-67, the company has also developed a rabbit polyclonal antibody product for ATRX detection suitable for glioma classification. The team hopes to follow with more antibodies for use in neuropathology and other applications. “We are increasingly investing in developing tumor markers, including mutations associated with other types of glioma,” Su explains.

left: IDH1 R132H (MRQ-67). right: ATRX (poly).
Growing value of markers in personalized medicine
As the trend of personalized medicine continues to expand, mutation markers are becoming increasingly valuable across the healthcare market. “We’ve already had some success with mutational markers for conditions such as Lynch syndrome,” says Heller. Lynch syndrome, also known as hereditary non-polyposis colorectal cancer, is the most common cause of hereditary colorectal cancer, which can also lead to skin cancer and other tumors, thus causing a significant burden.
“Patients with this diagnosis are often given their own treatment regimens that tend to yield better outcomes and better prognosis compared to other patients with colorectal cancer,” Heller said. We explain. This same concept shows potential for successful application in neuropathology. This is particularly important given the delicate nature of the brain and the obstacles this presents to the proposed surgical approach.
Heller concludes by sharing his vision for the future. Drugs can be precisely delivered and focused where the tumor is located. “