health
Many lung cancer patients now have access to potentially life-saving drugs.
Osimertinib, sold under the brand name Tagrisso, is available to patients with stage 1B to 3A lung cancer who have a specific genetic mutation and have had surgery to remove the cancerous tumor.
In these patients, Tagrisso was shown to reduce the five-year risk of cancer recurrence by up to 73% and the risk of death by up to 51%, according to study results published in the New England Journal of Medicine in the summer. It was done.
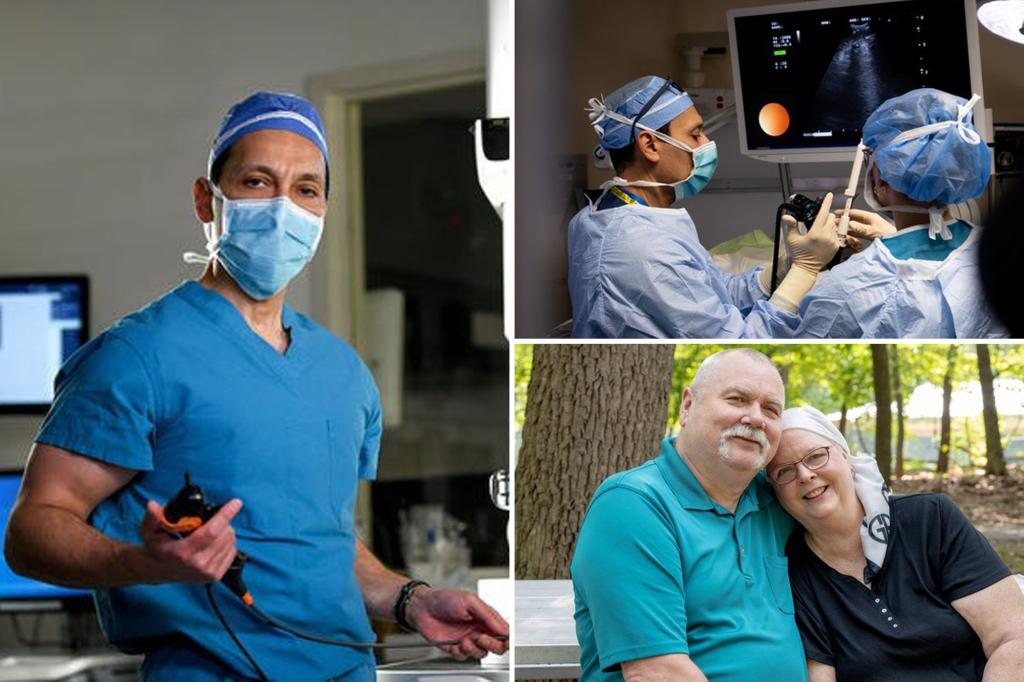
“This is a game-changer in the world of oncology,” Dr. Faiz Y. Vohra, chief of thoracic surgery and chief of central region surgery at Hackensack Meridian Health in New Jersey, told FOX News Digital.
“Previously, medical oncologists were satisfied with 5% or 10%, but now we’re talking about improved survival rates of over 50%.”
“This is a game-changer in the world of oncology,” Dr. Faiz Y. Vohra, chief of thoracic surgery and chief of central region surgery at Hackensack Meridian Health in New Jersey, told FOX News Digital.
“Previously, medical oncologists were satisfied with 5% or 10%, but now we’re talking about improved survival rates of over 50%.”
Dr. Bourla, who has prescribed the drug to several lung cancer patients, spoke of the “groundbreaking” results he has seen in his practice.
“We are truly in the era of personalized medicine,” Bourla also told FOX News Digital. “There are now a number of targeted therapies that are effective for patients with mutations in their tumors.”
For this particular drug, patients who have a gene mutation called EGFRm and have already had surgery are good candidates for Tagrisso.
“This pill helps prevent recurrence after surgical tumor removal in patients with genetic markers,” he said.
Patients with stage 4 lung cancer who have an EGFR mutation may also be eligible for the pill even if they haven’t had surgery, the doctor said.
To determine whether a patient has the mutation, a tissue sample is extracted from the tumor and tested. Usually results are obtained within 10-14 days.
Blood tests are also available, with results available within five to 21 days, Bourla said.
“About 25% of lung cancer patients will have an EGFR mutation,” the doctors estimated.
According to the American Cancer Society (ACS), approximately 238,000 new cases of lung cancer and 127,000 deaths are expected in 2023.
“We used to think of lung cancer as just a smoker’s disease,” says Bora. “We now know that more than 30% of people who develop lung cancer have never smoked, and the majority of them are women.”
Looking to the future, he hopes to be able to prescribe pills to patients immediately before other procedures or treatments, with the goal of shrinking tumors before surgery.
patient’s story
Kim Mosco, 67, was diagnosed with stage 2A lung cancer in February.
The mother of two underwent robotic surgery at Hackensack Meridian just a few weeks later.
“The lobectomy was performed by robotic surgery and was an incredible experience,” she told Fox News Digital. “I owe money to every doctor I have ever seen or consulted, and I will never be able to pay them back.”
Mosko then underwent four rounds of chemotherapy, which she completed at the end of June.
When Mosko’s doctors determined in July that he had a genetic mutation, they recommended he take Tagrisso.
“I didn’t need any convincing at all,” she told FOX News Digital. “I’m going to do whatever it takes to treat this cancer and prolong my life.”
Mosko has been taking Tagrisso for three and a half months now and plans to continue taking it daily for the next three years.
Her insurance covers the cost of the medication.
“Overall, my experience has been positive and I am grateful that this drug is available,” she said.
Mosko has experienced side effects such as a skin rash, diarrhea and fatigue, but “all of them are manageable,” she said.
“There’s no need to get your hopes up,” she added. “I absolutely believe that this drug will definitely prevent my lung cancer from coming back. I plan to live many more years.”
where to start
For lung cancer patients interested in Tagrisso, Dr. Bourla recommends seeing an experienced team of doctors and testing for genetic mutations.
“We consider every patient a candidate for treatment,” said Hackensack’s Bora.
With the advent of personalized treatments, he believes there is hope on the horizon for many lung cancer patients.
“This is a very optimistic time for patients with what was generally considered a deadly disease,” Dr. Bourla said.
“We are now in an era where in most cases we can either make it a chronic disease or aim for a complete cure.”
Safety information
AstraZeneca, the manufacturer of Tagrisso, indicates on its website that some side effects have been reported.
The most common include a low white blood cell count. Platelet count is low. Diarrhea; low red blood cell count (anemia). rash; muscle, bone, or joint pain. stomatitis. fatigue; cough; dry skin; changes in the nails, such as redness, tenderness, pain, inflammation, brittleness, separation from the nail bed, and nail shedding.
Although rare, serious side effects can occur that can affect the lungs, heart, eyes, skin, blood, and bone marrow.
AstraZeneca says patients who experience any troublesome or long-term side effects should talk to their health care provider.
You can also report side effects to the U.S. Food and Drug Administration by calling 1-800-FDA-1088.
Load more…
{{#isDisplay}} {{/isDisplay}}{{#isAniviewVideo}}
{{/isAniviewVideo}}{{#isSRVideo}}
{{/isSR video}}