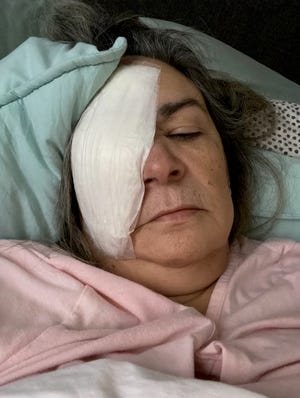
The September surgery to remove Clara Oliva’s infected right eye may be the reason she’s alive today.
It was her good eyes. What Oliva, 68, hoped was something that would allow her to continue with her normal grandmother’s job or continue her hairdressing career. She asked the doctors to save it.
Legally blind Oliva’s life changed 1,000%.
Oliva says nothing can restore her sight, but she hopes Lawsuit against maker of recently recalled eye drops Those associated with deadly outbreaks of Pseudomonas aeruginosa can provide accountability and prevent others from meeting the same fate.
EzriCare Eye Drops Recall Linked to Outbreak
Miami-based Oliva is suing India-based Global Pharma Healthcare, maker of EzriCare artificial tears prescribed for dry eyes last year. The complaint also includes the names of her Ezricare, EzriRx, and Aru Pharma, US-based distributors of drops.
of Eye drops recalled in early February The Centers for Disease Control and Prevention Outbreak of Pseudomonas aeruginosaAs of Monday, the drops were believed to have infected 68 people in 16 states.
According to the CDC, three people were reported dead, eight were reported blind, and four were reported to have had their eyeballs removed, with Oliva undergoing the procedure to have his eyeballs removed.
Pseudomonas aeruginosa is a rare and widespread drug-resistant bacterium not seen in the United States until last year, the CDC said.
“I couldn’t sleep because of the pain”
Authorities have been able to trace the current outbreak to the use of more than a dozen different brands of eye drops, but the most commonly reported brand used is preservative-free eye lubricant. EzriCare pointed out to be artificial tears.
Oliva said she was prescribed recalled eye drops in May to relieve dry eyes caused by contact lenses. Her insurance no longer covers the previous brand she used.
After months of using eye drops, on August 1, her right eye became swollen and red and was treated at a facility at Leon Medical Center in West Hialeah, Florida. Doctors diagnosed Oliva with a scar on her cornea and she prescribed a course of antibiotics. according to the lawsuit. Her eye condition worsened even though she was on medication.
“Urgent Threat”:CDC warns against Candida auris, a drug-resistant fungus invading healthcare facilities
Three days later, Oliva was rushed to the emergency room at the Bascom Palmer Eye Institute in Miami. According to the lawsuit, her doctor confirmed she had an ulcer in her eye, took a culture sample for her analysis, and increased the dose and frequency of the medication she was originally prescribed.
According to the lawsuit, Oliva was scheduled to go to the doctor 10 times during the month of August.
“I couldn’t sleep because of the pain,” Oliva said. She spent the whole day in the hospital.
Analysis of samples taken from her came back on August 10 and showed the presence of Pseudomonas aeruginosa.
A few weeks later, doctors began surgery to remove part of Oliva’s cornea and replace it with donor tissue. However, according to the lawsuit, the surgeon stopped the operation. The extent of infection was beyond the scope of treatment.
I had no choice but to remove Oliva’s eyes.
Other eye product recalls:Eye ointments recalled after previous eye drop recalls associated with outbreaks of infections, deaths
“On September 1, 2022, Mrs. Oliva’s right eye was surgically removed and replaced with a plastic implant,” the lawsuit states. “Mrs. Oliva is now legally blind given that the vision in her remaining left eye has fallen to her 20/200.”
Oliva continued to use EzriCare eye drops in her left eye until January when the clinic called and told her to stop using the drops and throw them away. Oliva said he was only told the drop was being recalled, but was not told why.
She and her family learned about possible Pseudomonas aeruginosa contamination when she heard about the recall on a television news segment.
“I want justice”
The lawsuit names six defendants.
- Global Pharma Healthcare Private Ltd. (Indian manufacturer)
- EzriCare, LLC (US distributor based in New Jersey)
- EZriRx, LLC (US distributor based in Delaware)
- Aru Pharma, Inc. (US distributor based in New York)
- Leon Medical Center, LLC. (Oliva’s healthcare provider)
- HealthSpring of Florida, Inc. (Oliva’s Health Insurance Plan)
Under Florida law, any party involved in the “commercial chain” or distribution of the product could be held liable, said Oliva’s attorney Ryan Yaffa.
“These groups manufacture, supply, promote, advertise, and sell products that are contaminated to people living in our country, and in our conditions that have reached our clients,” Jaffa said. . “This product reaches consumers, harms people, and people are seriously harmed.”
The lawsuits allege dozens in total, ranging from negligence to breach of express warranties.
Oliva is trying to recover medical bills and other damages.
“We’re not asking for a specific number at this point,” Jaffa said. “But as for her pain and suffering…we ask the jury to order what they think is fair and just.”
Oliva can’t pick up her grandchildren from school or indulge her passion for cooking. Instead, she spends her days relearning what were once basic tasks. How to walk without falling, how to bathe without assistance.
Simple pleasures that can be done at home, such as watching TV or reading a book, are not feasible.
Oliva moved from one son’s house to another. Because she was more spacious and could move around the house without accidentally bumping into things.
EzriCare faces multiple lawsuits, including two federal class actions. kentucky and new york.
USA TODAY’s inquiries to defendants in Oliva’s lawsuit were not immediately returned Wednesday.
“The people responsible for this have to pay. They have to pay for the damage they have done,” Oliva said. , life for the whole family…I want justice.