Soap company Dr. Bronner’s made headlines last year for offering psychedelic-assisted therapy to workers through its employee health insurance plan. Now, medical nonprofits that picked up this treatment are expanding its offering to patients across the country.
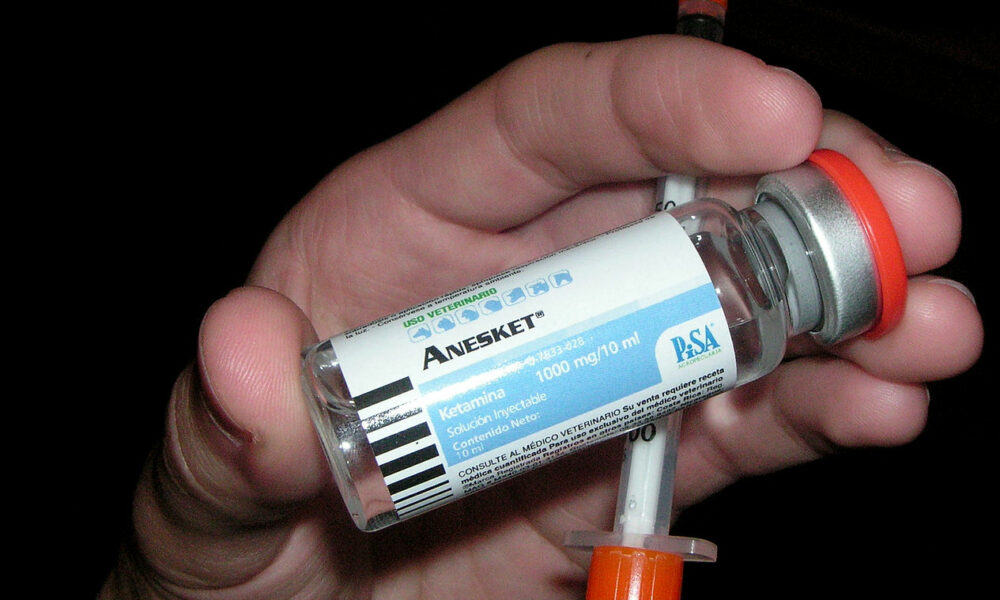
Enthea, which bills itself as “the first and only licensed provider of medical benefit plans to cover psychedelic-assisted therapy,” announced Wednesday that it is making ketamine-assisted therapy “nationwide available” as an available employee benefit. announced.
Employers interested in offering ketamine therapy as a covered treatment “simply add it as a supplemental benefit, similar to dental and vision care,” the company said.
“National availability is a pivotal moment in Enthea’s mission to help employers with workplace mental health issues,” said Enthea CEO and Co-Founder. said Sherry Rice. “Enthea’s services make it easier for companies to access this safe and effective treatment service for their employees, potentially impacting the millions of people in the United States living with a mental health condition. I’m proud of my gender.”
The company said in a press statement: release The expansion was made possible thanks to new partnerships that expand Enthea’s provider network, including Skylight Psychedelics and Innerwell.
In a partnership with Dr. Bronner’s in 2022, the soap company will offer “free ketamine-assisted therapy to all benefit-eligible employees” through Enthea, and approximately 7 percent of the company’s employees will receive benefits from their employer. This led to them signing up for the benefits offered. A study released last month found that employees who received ketamine treatment reported “dramatically improved mental health,” with generally positive outcomes.
Workers who completed therapy sessions supported by San Diego-based Flow Integrative found that dissociative drugs reduced symptoms of post-traumatic stress disorder by 86 percent, major depressive disorder by 67 percent, and anxiety disorders by 86 percent. He said his symptoms had improved by 65 percent.
“I am especially proud to partner with Entair to offer ketamine-assisted therapy to our employees,” said CEO David Bronner, a frequent supporter of drug reform. said at the time. “While not everyone experiences such profound healing, many of our team members report that their lives have dramatically improved as a result of ketamine-assisted therapy. We want to encourage businesses and organizations to also partner with Enthea and offer this benefit to their employees.”
Many experts already see the drug as an effective treatment option for conditions such as treatment-resistant depression and post-traumatic stress disorder. At the federal level, ketamine is approved by the Food and Drug Administration (FDA) for use as an anesthetic, and although it is not currently specifically approved for psychiatric disorders, physicians may not administer ketamine for off-label purposes. It is possible. Other medicines.
“Ketamine is an invaluable tool for therapists seeking to treat these diseases,” said Dan Rome, Enthea’s chief medical officer. “As programmed psychological defenses are lowered, more patients will be able to get to the root of their problems. This could lead to long-term symptom relief.
There is also an FDA-approved nasal spray called esketamine that has similar effects and is approved to treat treatment-resistant depression. Both esketamine and ketamine itself are classified as Schedule III of the Controlled Substances Act.
This regulatory situation could make coverage by mainstream insurance companies difficult or impossible, even though clinics administering the substance operate in states from Alabama to Washington. It means.
National Institute on Drug Abuse (NIDA) Director Nora Borkow said in 2021 that existing research on the benefits of ketamine for treatment-resistant depression is “eye-opening” and that more research is underway. Stated. “We have funded ongoing research into ketamine for opiate treatment and ketamine for pain.”
“We need to learn from what the evidence shows,” Borkow said. “If we can use ketamine in a safe way to treat severe depression, this would be a real-world example of how a drug that was thought to be dangerous can be used in a therapeutic way.”
Enthea says it plans to further expand its services to include MDMA- and psilocybin-based treatments “upon approval.” MDMA is expected to be reviewed by the Food and Drug Administration (FDA) next year after a successful Phase 3 clinical trial announced earlier this month.
The FDA defines MDMA as “groundbreaking treatmentResults of a new phase 3 study published in Nature earlier this month found that MDMA-facilitated talk therapy reduced symptoms in patients with moderate to severe PTSD. The randomized, double-blind, placebo-controlled trial involved 104 people with PTSD. The study found that the treatment was not only “generally well-tolerated” but also demonstrated robust treatment efficacy across participants.
In June, the FDA released its first-ever draft guidelines for psychedelic research.
Bronner, the soap company CEO, has been active in psychedelic and marijuana policy in recent years, including funding the New Approach PAC, which played a key role in getting psychedelic legalization measures on the ballot in Oregon and Colorado. We are working on this. The soap mogul also said at a press conference in July that he supports similar reform efforts in Arizona as well as Massachusetts.
Enthea said last month that the results of its ketamine partnership with Dr. Bronner’s motivated it to partner with wellness provider Nue Life to expand its treatment plans to cover telemedicine and home ketamine care.
California Governor decides on 17 marijuana and psychedelic bills by October 14th
Photo provided by: Wikimedia/Psychonaut.