– 13 hours ago
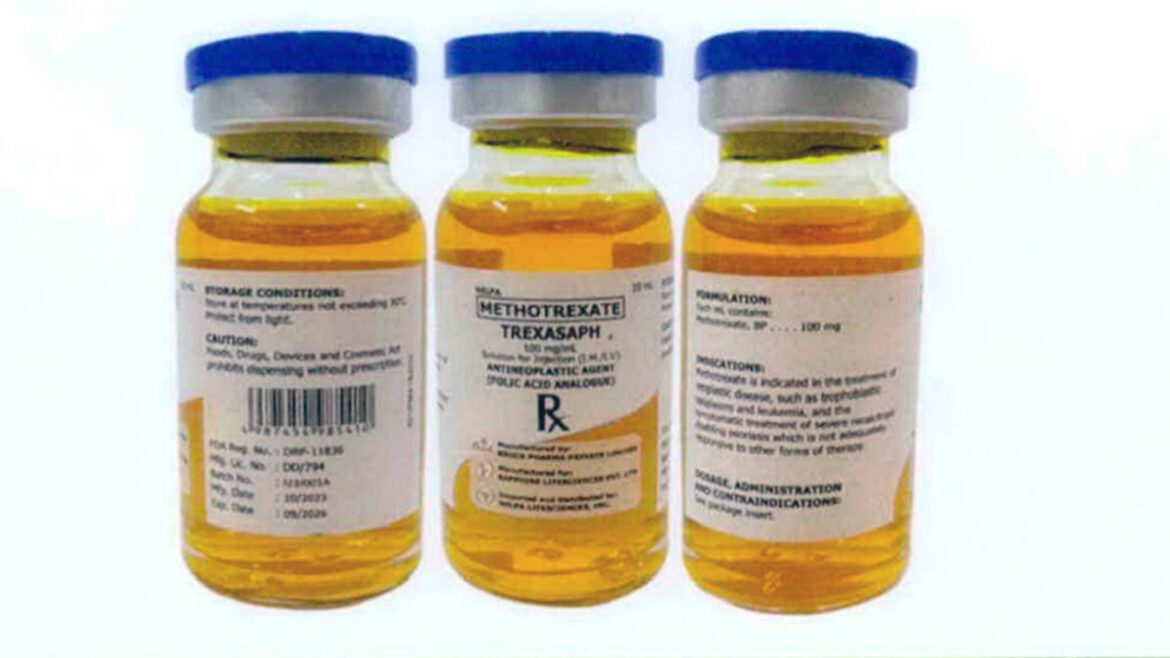
MANILA, Philippines — The Philippine Food and Drug Administration (FDA) has issued a second recall order after many lots of imported chemotherapy drug Trexaf were found to be contaminated with bacteria that can cause deadly infections. did.
Based on Agency Advisory No. 2024-0741 dated May 3, FDA Commissioner Dr. Samuel Zacate ordered a product recall of Trexaf (generic name methotrexate) 100 mg/mL Injection (IM/IV), batch number I23F025A. I23J001A, I23K002A, and I23H018A.
Methotrexate is a chemotherapy agent and immune system suppressant. It is used to treat neoplastic diseases (cancer) such as trophoblast neoplasms and leukemia, and for the symptomatic treatment of refractory and disabling psoriasis that does not respond adequately to other treatments.
Read: FDA orders recall of chemotherapy injections
Patients receiving methotrexate treatment may have a weakened immune system and be more vulnerable to opportunistic infections.
“Following reports of adverse reactions in pediatric patients receiving Trexaf, [FDA] Laboratory analysis of the collected samples was carried out. “Trexaf, batch number I23J001A, failed sterility testing and tested positive for Pseudomonas aeruginosa, indicating product contamination,” the FDA said.
Pseudomonas aeruginosa is usually transmitted by patients who have been hospitalized for more than a week. It can cause a bloodstream infection (sepsis), which can be fatal. Any product that is contaminated and administered directly to the body can pose a significant risk to the patient.
This bacteria grows in a variety of ways, including through water (from sinks, bathtubs, pools, hot tubs, humidifiers, and kitchens), soil, food, and contaminated medical equipment (such as ventilators and urinary catheters). and spread.
Pseudomonas aeruginosa infections are spread from person to person, usually from contaminated surfaces or hands.
“Jurisdiction”
Regulators have directed distributors, hospitals, retailers, pharmacies, and clinics to stop further distribution, sale, or use of Trexaf.
Consumers are advised not to use or purchase the affected products.
“All local governments and law enforcement agencies are asked to ensure that affected product lots are not sold or made available in their localities or jurisdictions,” the FDA said.
Based on the FDA’s database, Trexaf is manufactured by India-based Bruck Pharma Private Ltd. and imported by Nelpa Lifesciences Inc. of Parañaque City.
It has been registered with the FDA since May 2022, and its registration is scheduled to expire in May 2027.
This is the second time this year that the FDA has issued a recall order for Trexaf.
In March, the FDA warned all government and private health care facilities to stop selling, dispensing, and using Trexaf after samples belonging to batch number I23J001A tested positive for Pseudomonas aeruginosa. issued a recommendation.
To report the continued sale or distribution of affected batches of Trexaf, the public can email FDA via ereport@fda.gov.ph or call the Center for Drug Regulatory Research at 02 You can also call ). 8809-5596.
Mr. Shapril also reflected on this.
The FDA also ordered a recall of Lot No. 29080, Shapril (generic name enalapril maleate) 10 mg tablets, because they contained less of the active ingredient than advertised.
“Laboratory analysis conducted by the FDA determined that the particular lot contained only 8.54 mg of enalapril maleate per tablet,” Zacate said in FDA Advisory No. 2024 dated May 3. -0740 stated.
Shapril is manufactured by Scheele Laboratories Philippines, Inc., based in Valenzuela City.
Enalapril maleate is an angiotensin-converting enzyme inhibitor used to treat high blood pressure (high blood pressure) and can help prevent strokes, heart attacks, and kidney problems.
It may also be given to patients with certain cardiac problems (including asymptomatic left ventricular dysfunction) to delay the onset of heart failure and reduce the incidence of coronary ischemic events such as heart attacks. There is also.