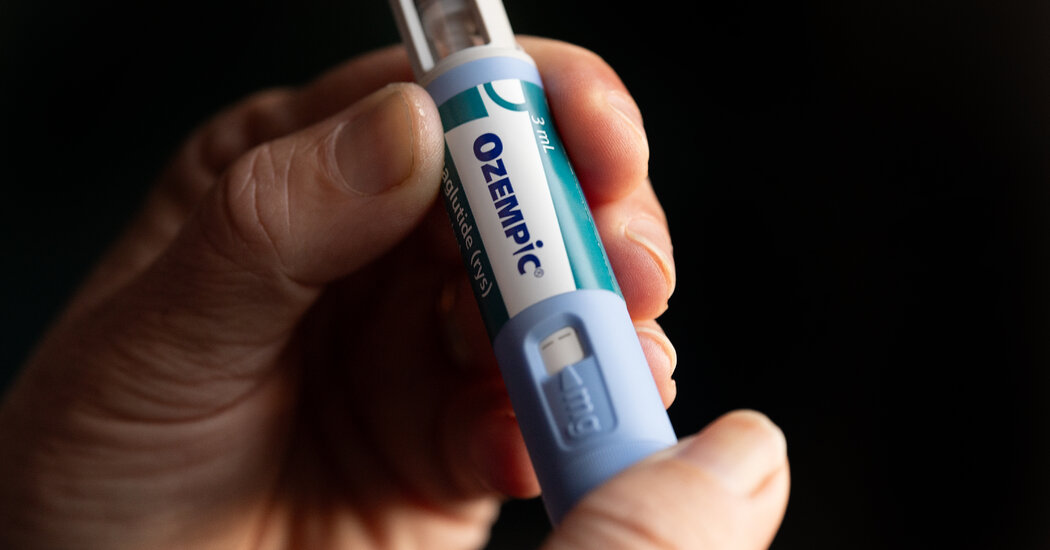
The Food and Pharmaceutical Bureau on Tuesday has approved Ozenpicks to reduce the risk of serious complications of both type 2 diabetes and chronic renal disease.
Type 2 diabetes is the main cause of chronic renal diseases and affects one or more in seven adults in the United States. This drug can reduce the possibility of both patients in the kidney disease, renal failure, and death due to cardiovascular issues.
“In the past 20 years, researchers have worked hard, but they were almost delivered,” said Dr. Stephen Gough, a senior vice president of NOVO NORDIRS, a production of Ozempic. 。 “It is really exciting to have such a new thing and provide promises to patients,” he said.
Ozempic has already been approved to treat type 2 diabetes and reduce the risk of major cardiovascular events in adults who have a history of diabetes and heart disease. FDA has been approved by Ozen pick for research that shows that people with type 2 diabetes and chronic kidney disease are 24 % lower than those who need dialysis and transplantation. Based on the decision to expand. The person who took placebo. They were also slow to decline in the kidneys and were unlikely to die due to cardiovascular issues.
“If the kidney function can be reduced, the better, the better,” said Dr. Honich, a kidney specialist at Beston’s Bes Israel Debones Medical Center. Dr. Hoenig said she had already prescribed Ozempic to some of her patients.
Doctors have long been dissatisfied with how few treatment options are. Many patients take medicine to reduce blood pressure, blood sugar, and cholesterol, which tends to rise in chronic kidney disease. The patient also takes drugs to relieve the swelling generated when the kidneys can not filter the liquid properly, and take drugs that manage iron, calcium, and vitamin D levels.
Thank you for patience while confirming access. If you are in reader mode, finish the time and log in. Or subscribe to all time.
Thank you for patience while confirming access.
Are you already a subscriber? Login.
Do you want all time? Subscribe.