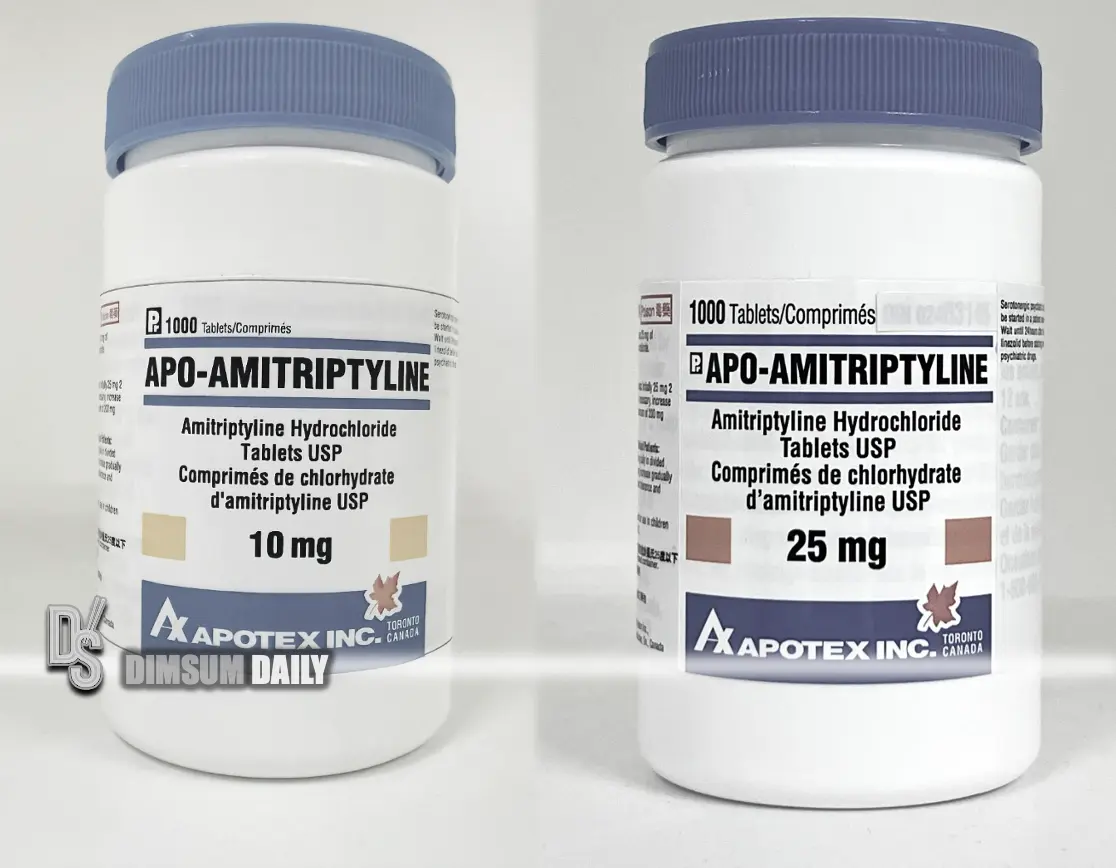
18 October 2024 – (Hong Kong) The Department of Health (DH) today announced that it has approved the recall of 14 lots of Apoamitriptyline tablets from Hind Wing Co Ltd, a licensed pharmaceutical wholesaler. This precaution follows the detection of known impurities. It is used in products as N-nitrosonortriptyline (NNORT).
Affected products include Apoamitriptyline tablets in both 10mg and 25mg formulations, each with a specific batch number. Based on clinical test results, NNORT has been classified as a possible human carcinogen, and overseas manufacturers have begun a recall.
DH has received reports that these batches, which are used to treat depression, were imported into Hong Kong and distributed to DH clinics, pharmacies, private doctors and private hospitals, as well as being re-exported to Macau.
Hind Wing has set up a hotline (2541 5731) for inquiries regarding the recall.
A DH spokesperson said: “To date, we have not received any reports of side effects related to the affected products. DH will be monitoring the situation closely.”
Additionally, the spokesperson advised patients currently taking these drugs not to suddenly stop using them and to consult a medical professional for proper guidance and management.