The Drug Enforcement Administration made the announcement Wednesday, 16 years in the making. There will be a special registration process for prescribers who want to provide controlled substances, such as opioids and stimulants used to treat ADHD, via telemedicine.
The move would finally fulfill a mandate the DEA has largely ignored since Congress first enacted the program in 2008. But the new special registration system is just a proposal, meaning the incoming Trump administration could abolish it.
It also had many limitations and immediately caused a backlash. Among other rules, health care providers seeking to prescribe Schedule II drugs, including Ritalin and Adderall, must physically reside in the same state as the patient. It would also require at least 50% of prescriptions to be issued after an in-person consultation, a potential threat to health care providers whose core business is telemedicine.
The Alliance for Connected Care, an industry group representing telehealth providers, said in a statement that it is “very pleased with the language in the proposed rule governing what portions of patient care can be provided via telehealth. “We are concerned about this because this is not an adequate guardrail for telemedicine.” Telemedicine services. Similarly, limiting the geographic areas in which telehealth can be provided undermines the value of creating virtual access for patients who need it most. ”
The DEA’s new regulations, issued with just days left in President Biden’s term, are the latest chapter in a saga that dates back to the passage of a law regulating online pharmacies in 2008. The law included a requirement that the DEA create a special registration process for practitioners who wish to prescribe controlled substances remotely.
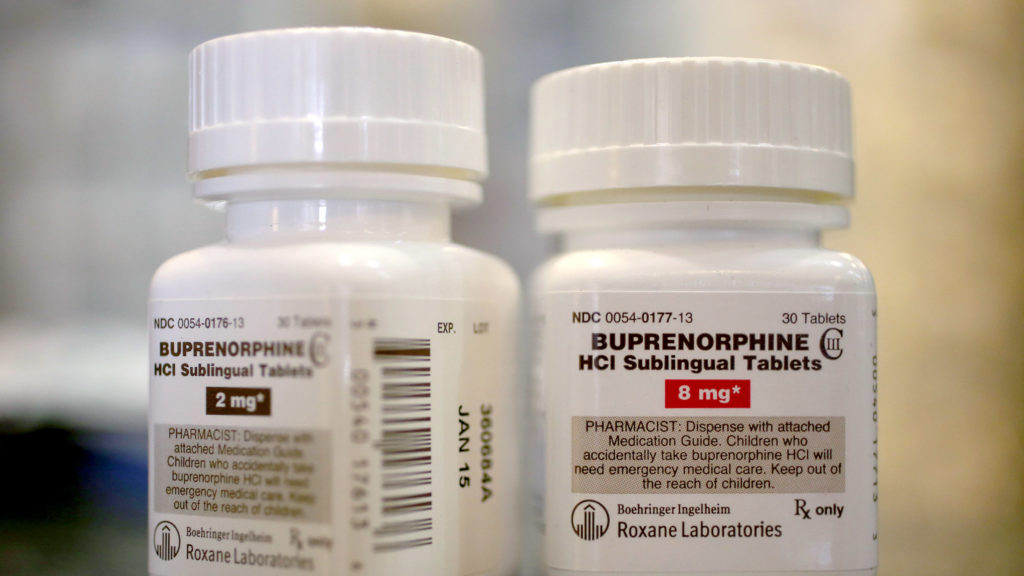
Emergency regulations enacted at the beginning of the coronavirus disease (Covid-19) pandemic significantly increased flexibility in telemedicine prescribing of controlled substances. But the DEA’s efforts to enact new post-pandemic regulations have faced significant resistance, particularly regarding buprenorphine, a Schedule III drug commonly used to treat opioid addiction.
In another rule, the DEA moved to allow prescribers to continue providing six months’ worth of buprenorphine without an in-person visit. The final version of the buprenorphine-specific rule, which is scheduled to go into effect in mid-February, means it will be much harder for the Trump administration to overturn.
After six months, patients will be able to refill prescriptions through in-person visits, and as the DEA’s broader telemedicine proposal is finalized, prescribers will be able to refill their prescriptions remotely if they are specifically enrolled in the new system. You will be able to receive refills. Under the new system, pharmacists will be responsible for checking patient identification upon collection.
However, pharmacies remain a major hurdle for many patients seeking buprenorphine. Beyond the discrimination that many addicts face in retail stores, many major pharmaceutical retailers simply do not have inventory medicine.
The DEA’s new rules create a link between buprenorphine, commonly known by its trade name “Suboxone,” and other controlled substances, amid growing recognition that the drug is highly unlikely to cause an overdose. It makes many distinctions. In October, two Democratic senators introduced a bill that would effectively direct the DEA to stop monitoring buprenorphine altogether until the opioid crisis is over.
Drugs such as buprenorphine and methadone, Schedule II drugs that doctors cannot directly prescribe to patients to treat addiction, are seen as key weapons in the U.S. response to the opioid crisis but remain heavily criticized. I am receiving it.
For example, buprenorphine prescribers are only required to check the prescription drug monitoring database of the patient’s state. In contrast, if the broader proposal is finalized, prescribers seeking special registration to provide other controlled substances through telemedicine will be required to use all existing monitoring systems across all 50 states and U.S. territories. However, this provision will not come into force until three years from now. Regulations have been finalized.
“The DEA published these rules not because it was fully ready to implement them, but to ensure they would not be waived by the incoming Trump administration,” said Foley & Lardner, a law firm considered an authority on telemedicine. said attorney Marika Miller. problem. “The long-awaited special registration process has been met with disagreements with stakeholders, and further notice-and-comment rulemaking is expected on related rules, among other concerns. A key issue for stakeholders involved in both regulations is the national prescription drug monitoring program check requirement, and the DEA appears to continue to underestimate this burden.
Currently, health care providers are relying on the DEA’s repeated extensions of coronavirus-era telehealth flexibilities, which the agency recently extended through the end of 2025. However, timelines for finalizing regulations are generally long, and even if the rules move forward, it is unclear whether the DEA will be able to finalize them by the end of the year. Without further extension, the new rules risk creating a new “telemedicine cliff” for patients who rely on drugs prescribed via telemedicine.
The DEA’s future also remains in flux. President-elect Donald Trump announces his nominee to lead Florida’s Hillsborough County Sheriff Chad Chronister after his original nominee resigned amid criticism of his enforcement of coronavirus lockdowns. I haven’t. Measures for 2020.
Mario Aguilar contributed reporting.
STAT’s chronic health coverage is supported by a grant from. bloomberg philanthropy. our financial supporter It has no role in any of our journalism decisions.