Popular prescription anxiety medications include: recalled nationwide Because there was a “potentially life-threatening” mistake on the carton.
Pharmaceutical manufacturer based in Pennsylvania Endo announced this week The company announced that it is expanding its recall of clonazepam tablets because some cartons have the wrong strength and wrong drug number.

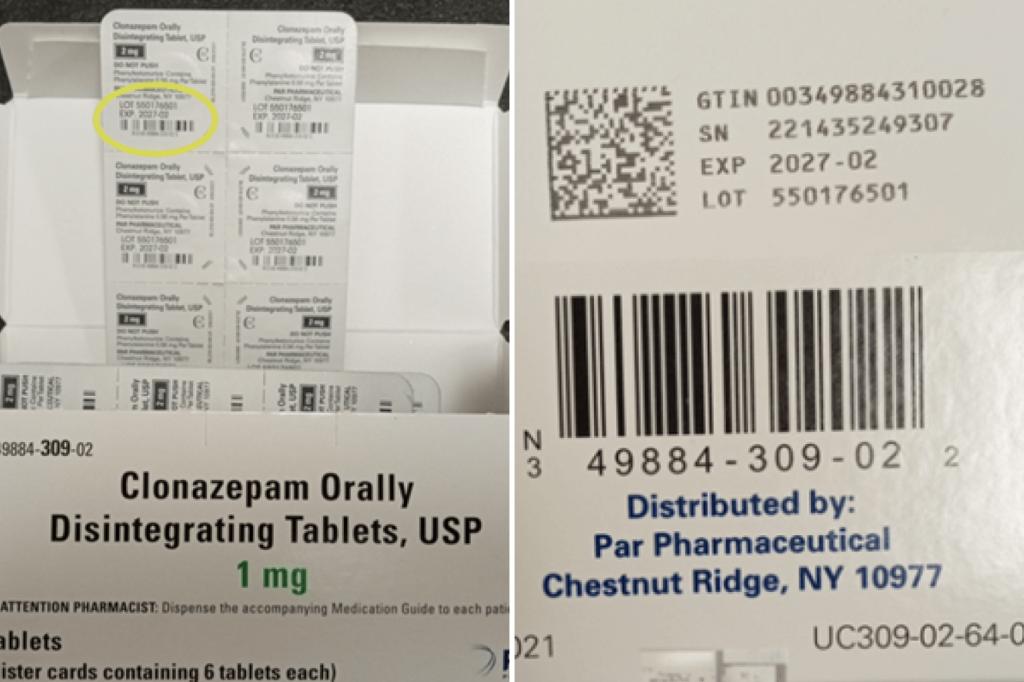
The new recall affects 16 lots of Clonazepam orally disintegrating tablets USP (C-IV) in doses ranging from 0.125 milligrams to 2 milligrams. The package contains 10 blister strips, each containing 6 tablets. Valid from August 2026 to February 2027.
Clonazepam is a benzodiazepine used to treat panic disorder and certain types of seizures.
Professor Endo warns that taking high doses of clonazepam may increase the risk of drowsiness, confusion, dizziness, decreased reflexes, and decreased muscle control and strength.
There is also a risk of “serious, possibly life-threatening” breathing difficulties, especially for people with respiratory conditions, those prescribed maximum doses, or those taking other medications that affect breathing. there is.
Endo reported that as of Monday, he had not received any reports of problems resulting from the recall. pharmaceutical company First recall announced in July Just one lot of clonazepam.
At the time, Endo blamed the mislabeling on “an error by a third-party packager.”
Some cartons displayed product strength as 0.125 mg instead of 0.25 mg. Endo said the blister strip inside the package reflects the correct strength.
The recalled boxes also list New York-based Parr Pharmaceuticals as a distributor. The Chestnut Ridge-based company sold clonazepam before the product was acquired by Endo.
If you have questions about the recall, please call (855) 589-1869 or email [email protected].
Retailers who carry these products have been instructed not to sell them, and consumers should not consume them. If you think you have taken the wrong dose of clonazepam, contact your doctor.