overview: A new stem cell therapy approach eliminates established brain tumors, provides long-term immunity, and trains the immune system to prevent cancer from returning.
sauce: Brigham and Women’s Hospital
Scientists are using new methods to turn cancer cells into powerful anticancer drugs.
In the latest study from the lab of Khalid Shah, MS, Ph.D. at Brigham and Women’s Hospital, a founding member of the Mass General Brigham Health Care System, researchers found that established tumors could be eliminated and long-term survival was possible. developed a novel cell therapy approach to induce It trains the immune system so that it can prevent the cancer from coming back.
The team tested a dual-acting, cancer-killing vaccine in an advanced mouse model of the deadly glioblastoma of the brain, with promising results.
The survey results are Science Translational Medicine.
“Our team pursued a simple idea: to take cancer cells and turn them into cancer killers and vaccines,” says Stem Cell and Translational Immunotherapy. said Khalid Shah, MS, Ph.D., Director of the Center (CSTI) and corresponding author. Associate Director of Neurosurgery, Brigham University, Harvard He Medical School and faculty member of the Harvard Stem Cell Institute (HSCI).
“We are using genetic engineering to develop therapies that repurpose cancer cells to kill tumor cells, stimulate the immune system to destroy primary tumors, and prevent cancer.”
Cancer vaccines are an area of active research in many laboratories, but the approach taken by Shah and his colleagues is unique. Instead of using inactivated tumor cells, the team reuses live tumor cells with abnormal characteristics. Like a homing pigeon returning to its roost, live tumor cells travel long distances in the brain to return to the location of their fellow tumor cells.
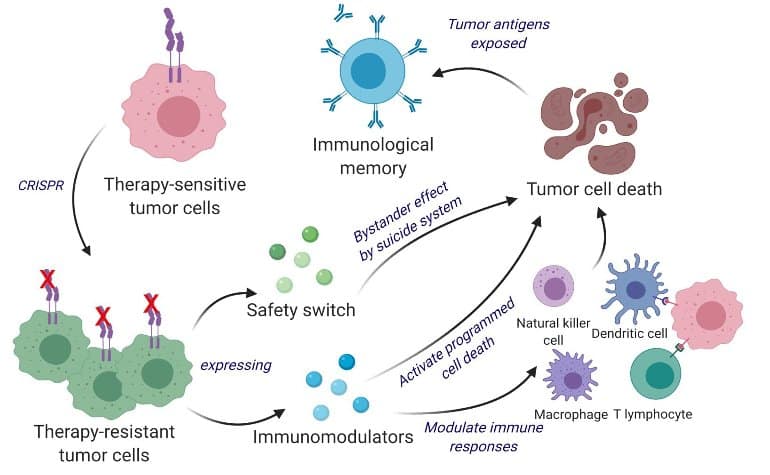
Taking advantage of this unique property, Shah’s team manipulated living tumor cells using the gene-editing tool CRISPR-Cas9 and repurposed them to release tumor cell killing agents.
In addition, engineered tumor cells are designed to express factors that enable the immune system to easily discover, tag and remember, priming the immune system for long-term anti-tumor responses. .
The team has developed repurposed, CRISPR-enhanced and reverse-engineered therapeutic tumor cells (ThTCs) in a variety of mouse strains, including those that yield human-derived bone marrow, liver, and thymocytes that mimic the human immune microenvironment. ) was tested. Shah’s team also incorporated his two-layer safety switch into cancer cells. When this switch is activated, he will eradicate ThTC if necessary.
This dual-acting cell therapy is safe, applicable, and effective in these models, suggesting a roadmap to therapy. Further testing and development is needed, but Shah’s team specifically chose this model and used human cells to smooth the way for translating their findings into the patient setting.
“Throughout everything we do at the center, we never lose sight of our patients, even if they are highly technical,” says Shah.
“Our goal is to employ innovative yet translatable approaches that will ultimately allow us to develop therapeutic cancer-killing vaccines that will have a lasting impact on medicine.”
Shah and colleagues note that this therapeutic strategy is applicable to a broader range of solid tumors, requiring further investigation of its application.
About this Brain Tumor Research News
author: press office
sauce: Brigham and Women’s Hospital
contact: Press Office – Brigham and Women’s Hospital
image: Image credits Kok Siong Chen and Khalid Shah
Original research: open access.
“A bifunctional cancer cell-based vaccine simultaneously promotes direct tumor killing and anti-tumor immunity’ Kok-Siong Chen et al. Science Translational Medicine
overview
A bifunctional cancer cell-based vaccine simultaneously promotes direct tumor killing and anti-tumor immunity
Administration of inactivated tumor cells is known to induce potent anti-tumor immune responses. However, the efficacy of such approaches is limited by their inability to kill tumor cells before inducing an immune response. Unlike inactivated tumor cells, live tumor cells have the ability to track and target tumors.
Here, we have developed a bifunctional whole-cancer cell-based therapy with direct tumor-killing and immunostimulatory roles. Recycle interferon-β (IFN-β)-sensitive to resistant tumor cells by knocking out IFN-β-specific receptors using CRISPR-Cas9, followed by the immunomodulatory agents IFN-β and granules We engineered them to release sphere-macrophage colony-stimulating factor.
These genetically engineered therapeutic tumor cells (ThTCs) induce caspase-mediated cancer cell apoptosis, downregulate platelet-derived growth factor receptor β expressed by cancer-associated fibroblasts, and enhance anti-tumor immunity. We eliminated established glioblastoma tumors in mice by activating cell trafficking and antigen specificity. T cell activation signaling.
ThTC efficacy based on this mechanism translated into survival benefit and long-term immunity in primary, recurrent, and metastatic cancer models in immunocompetent and humanized mice. Incorporation of a dual kill switch into ThTC involving herpes simplex virus 1 thymidine kinase and rapamycin-activated caspase-9 ensured the safety of this approach.
Arming naturally neoantigen-rich tumor cells with bifunctional therapeutics represents a promising cell-based immunotherapy against solid tumors and establishes a roadmap to clinical translation.