Overview
Background
G protein-coupled receptor, family C, group 5, member D (GPRC5D) is an orphan receptor expressed on malignant plasma cells. Tarquetamab, a bispecific antibody against CD3 and GPRC5D, redirects T cells to mediate killing of GPRC5D-expressing myeloma cells.
method
Download the research summary PDF.
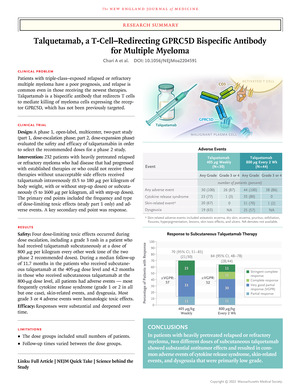
The phase 1 trial investigated whether tarketamab was administered intravenously weekly or biweekly (doses ranging from 0.5 to 180 mcg/kg body weight) or subcutaneously every week, biweekly, or monthly (5 to 1600 mcg/kg). I rated it. If relapsed or refractory multiple myeloma has been severely treated with prior therapy and has progressed on established therapy (median of 6 prior therapies), or if there are no unacceptable side effects were unable to receive treatment for The primary endpoints of frequency and type of dose-limiting toxic effects (study part 1 only), adverse events, and laboratory abnormalities were assessed to select recommended doses for Phase 2 studies.
result
At the time of data cutoff, 232 patients were receiving tarketamab (102 intravenous and 130 subcutaneous). Two subcutaneous doses recommended in Phase 2 studies (405 μg per kilogram weekly) [30 patients] and 800 μg per kilogram every other week [44 patients]), common adverse events were cytokine release syndrome (77% and 80% of patients, respectively), skin-related events (67% and 70%), and dysgeusia (63% and 57%). All but one cytokine release syndrome event were grade 1 or 2. His one dose-limiting toxic effect of grade 3 rash was reported in a patient who received talquetamab at the 800 μg dose level. At a median follow-up of 11.7 months (patients receiving tarquetamab at the 405 mcg dose level) and 4.2 months (patients receiving tarquetamab at the 800 mcg dose level), the proportion of patients who demonstrated a response was 70 % (95% CI [CI], 51–85) and 64% (95% CI, 48–78), respectively. The median duration of response was 10.2 months and 7.8 months, respectively.
Conclusion
Cytokine release syndrome, skin-related events, and dysgeusia were common with tarquetamab treatment, but were mainly of low grade. Tarketamab has produced substantial responses in heavily pretreated patients with relapsed or refractory multiple myeloma. (Funded by Janssen Research and Development; MonumentTAL-1 ClinicalTrials.gov number, NCT03399799.)