Biosimilars are a hot topic at the 2023 American Society of Health System Pharmacists (ASHP) Summer Conference and Exhibition.1 “[I think] this will be a hot topic [for the rest of the meeting,” said Courtney Queen, PharmD, CSP, of the University of Kentucky Specialty Pharmacy and Infusion Services in Lexington, Kentucky, during her presentation on the specialty pharmacy pipeline.
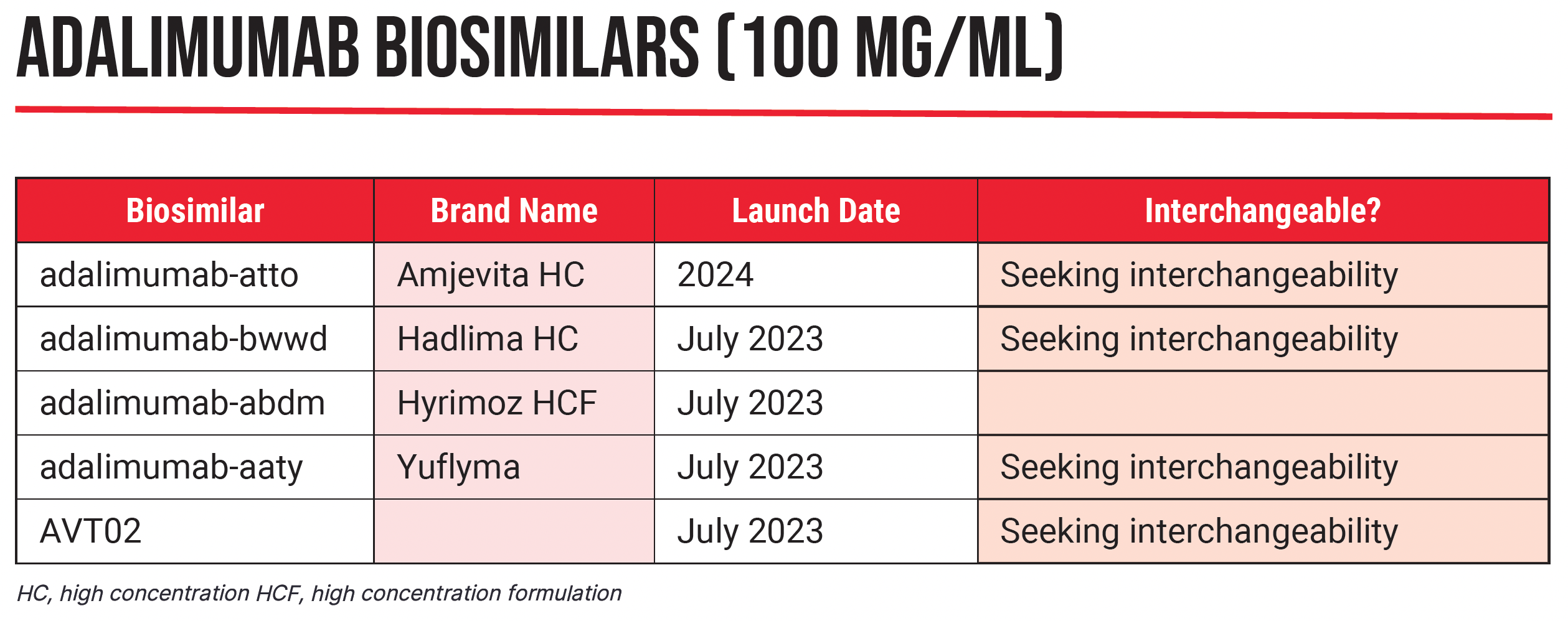
Over the next 5 years, multiple biosimilars medications are set to join the 41 that have already gained FDA approval, including biosimilar products to ustekinumab (Stelara), golimumab (Simponi), certolizumab pegol (Cimzia), and etanercept (Enbrel). The bulk of biosimilars being released in 2023, however, represent significant competition to behemoth anti-tumor necrosis factor-a monoclonal adalimumab (Humira), approved to treat a slew of conditions including rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis, among others.
The flood began as a trickle in January 2023 with the launch of adalimumab-atto (Amjevita), approved in 2016 as the first adalimumab biosimilar. By July 31, adalimumab biosimilars adalimumab-abdm (Cyltezo), the first interchangeable adalimumab biosimilar; adalimumab-adaz (Hyrimoz); and adalimumab-aqvh (Yusimry), will join the list of available products.

“largely [adalimumab biosimilar] The product is citrate-free, which is a big thing,” said Queen, citing the patient-friendliness of current citrate-free adalimumab products.
Several high-concentration products are due to be released in July this year, none of which are citrate-free. “These will be more similar to what patients are currently accustomed to, with lower doses and higher concentrations,” Queen said.
Another issue surrounding adalimumab biosimilars is pricing. “I know we’re all interested in what the plans look like,” Queen said. Adalimumab-at is currently available at prescription prices comparable to adalimumab. But as more adalimumab biosimilars are launched, Queen wants to know how this pricing structure will change and whether these products will be priced closer to generics.
Compatibility of these products is still an open question, and laws and guidelines vary from state to state. “Compatibility will potentially open opportunities.” [a specific biosimilar] This is because to gain greater market share, you will automatically replace brand name products, whether or not they are developed. [biosimilars] You will have to write your name. “
With a large number of adalimumab biosimilar products coming to market soon, providers should keep the patient’s perspective in mind. “What else is different?” she asked. Aside from pre-approval and other payer-related concerns, it’s important to consider the most patient-friendly product. “Think of rheumatoid arthritis patients who have limited hand function. [autoinjector pen size]’ and prefer smaller pen devices that are easier to use. “It will be very interesting to look at all the different products and see what little nuances they have.”