– 3 hours ago
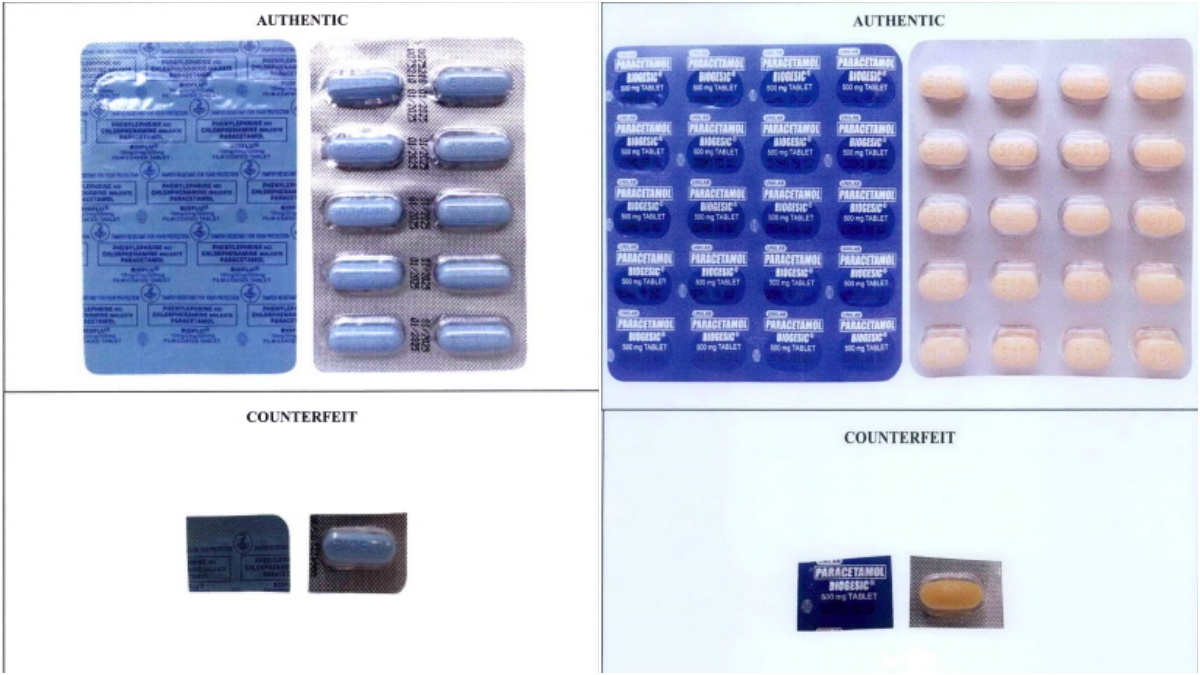

Fakes vs. the real thing Drug company Unilab says counterfeit pills may be too pale, too dark or abnormal in color when compared to the real thing. —Food and Drug Administration photo
MANILA, Philippines — The Food and Drug Administration (FDA) has warned the public to be cautious when buying over-the-counter (OTC) medicines after it discovered counterfeit versions of common drugs manufactured by local pharmaceutical companies.
In two recent advisories issued on May 17, FDA Commissioner Samuel Zacate reported the discovery of counterfeit products of Cremil S, Araxan FR, Biogesic, Medicol Advance, Bioful and Tuseran Forte, all manufactured by Unilab.
Cremil S (aluminum hydroxide + magnesium hydroxide + simethicone) is used to relieve stomach pain caused by excess stomach acid.
Meanwhile, for headaches and body aches, Alaxan FR (ibuprofen + paracetamol), Biogesic (paracetamol) and Medicol Advance (ibuprofen) are taken.
Bioflu (phenylephrine hydrochloride + chlorpheniramine maleate + paracetamol) and Tuseran Forte (dextromethorphan hydrobromide + phenylpropanolamine hydrochloride + paracetamol) are used to treat cough, cold, fever and other flu symptoms.
Read: FDA warns of growing number of counterfeits of popular drugs
Authorized stores only
“All healthcare professionals and the public are warned that counterfeit medicines exist in the marketplace that pose potential risks and injuries to consumers, and consumers are reminded to purchase medicines only from FDA-approved facilities,” the FDA said.
Read: FDA steps up crackdown on counterfeit drugs sold online
Selling counterfeit drugs is punishable under Republic Act No. 9711 (FDA Act of 2009) and RA 8203 (Special Law on Counterfeit Drugs). Anyone found in possession of counterfeit drugs may be subject to imprisonment of not less than six months and one day.
Unilab on Monday also issued a warning against counterfeit medicines and vitamins, which it said may contain dangerous substances such as chalk, cornstarch, flour, pollen, rat poison, arsenic and cement instead of active pharmaceutical ingredients.
“Most fake drugs [for] The real product could put patients at risk if they were to ingest it. [of] “It is possible that the product may not provide the desired benefits, such as managing symptoms or treating the disease,” the company said in a post on its official Facebook page.
“[They may also] Ingesting toxic substances… [over] Over a long period of time, [result in] “It can cause serious health conditions that can lead to hospitalization and death,” he added.
Read: FDA warns against unregistered Vitabears supplement
How to spot a fake
To spot counterfeit medicines, Unilab urges consumers to look out for products that are too light or dark in color or look abnormal compared to the real thing. Counterfeit medicines may be the wrong size or shape, have an odd odor, or be moldy or dirty.
Other things to look out for include misspelled or grammatical words on the packaging, missing expiration dates or lot numbers, etc. The packaging or security seals may also be dirty, tampered with, damaged, or printed on substandard materials.
Unilab officials previously said in interviews that law enforcement had found the counterfeit drugs being sold at retailers that did not have a license from the FDA to sell them.
In 2022, the Ministry of Interior and Local Government instructed local governments responsible for issuing business licenses to retail stores to pass ordinances banning the sale of medicines in such establishments across the country.
This comes after the FDA received a total of 185 reports in just one month of retailers illegally selling medicines, nine of which were selling counterfeit medicines, including medicines to treat COVID-19.

